ANSWER
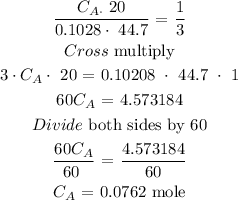
The molarity of the unknown phosphoric acid is 0.0762 moles
Explanation
What to find? The molarity of the unknown phosphoric acid
Given parameters
Volume of base = 44.7mL
Concentration of base = 0.1028 mole
Volume of acid = 20.0mL
nA = 1
nB = 3
To find the concentration of the unknown phosphoric acid, we need to write the balanced equation for the reaction.
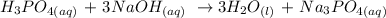
From the equation of the reaction; This means that 1 mole of H3PO4 neutralizes 3 moles of NaOH
To find the molarity of H3PO4, we will need to apply the below formula
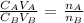
Where;
CA = Concentration of the acid
VA = Volume of acid
CB = Concentration of the base
VB = volume of the base
nA = mole ratio of acid
nB = mole ratio of base
Substitute the parameters into the above formula