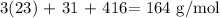Answer:
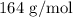
Step-by-step explanation:
Here, we want to get the molar mass of the given molecule
To calculate this, we need the atomic masses of the individual atoms
We have 3 atoms present:
For Sodium (Na), the atomic mass is approximately 23 amu
For Phosphorus, the atomic mass is approximately 31 amu
For Oxygen, the atomic mass is approximately 16 amu
We have 3 atoms of sodium, 1 atom of phosphorus, and 4 atoms of oxygen
Adding the values, we have it that: