The balanced equation of the reaction described is:
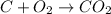
Now, to find the mass of CO2 produced, we will follow these steps:
1. We are told that after the reaction there are 14.1 g of oxygen left unreacted, that is, the grams of oxygen that react will be 46.1g - 14.1g =32 g O2. We calculate the moles that react using the molar mass of oxygen. Molar mass oxygen= 31.998gO2
2. We find the moles of CO2 by stoichiometry
3. We find the mass of CO2, using the molar mass of CO2. Molar mass CO2=44.01g/mol
Let's proceed with the calculations.
1. Moles of O2 that react
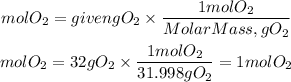
2. Moles of CO2 produced
By stoichiometry, the ratio CO2 to O2 is 1/1. So, the moles of CO2 produced is:
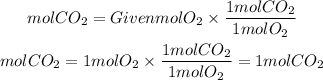
3. Grams of CO2 produced
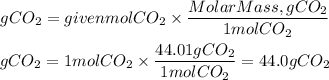
Answer: The mass of carbon dioxide produced was 44.0g