ANSWER
The mass of H2O that reacted is 65.88 grams
Step-by-step explanation
Given that;
The volume of oxygen atom at STP is 40.9L
Follow the steps below to find the mass of water reacted
Step 1; Write the balanced equation of the reaction
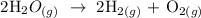
Step 2; Find the number of moles of oxygen atom
Recall, that 1 moles of a gas at STP is equivalent to 22.4 L/mol
Let x represents the number of moles of oxygen
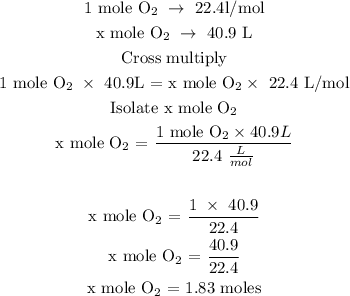
The moles of O2 is 1.83 moles
Step 3; Find the number of moles of H2O using a stoichiometry ratio
Let x represents the number of moles of H2O
In the given reaction, 2 moles of water give 1 mole of O2
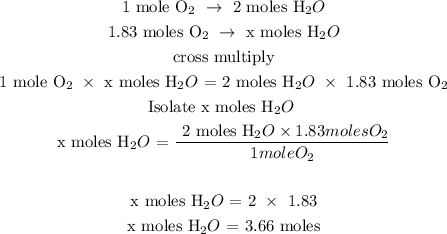
The number of moles of H2O is 3.66 moles
Step 4; Find the mass of H2O using the formula below
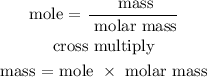
Recall, that the molar mass of water is 18.0 g/mol
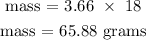
Therefore, the mass of H2O that reacted is 65.88 grams