Answer:
The pH of the solution is 4 and it is acidic.
Step-by-step explanation:
To calculate the pH knowing the concentration of H3O+, it is necessary to use the pH formula:
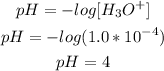
So, the pH of the solution is 4. Since the pH value is less than 7, the solution is acidic.