The question requires us to calculate the amount of heat released in the methane combunstion reaction when 32 g of methane are burned.
The first step in this question is balance the reaction:
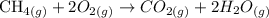
Now that we have the balanced reaction, we need to calculate the molar mass of methane in order to have the amount of moles that will be burned. To calculate the molar mass, we conisder the atomic mass of carbon (12 u) and hydrogen (1 u) and the number of these atoms in the molecule:
molar mass (CH4) = (1 * 12) + (4 * 1) = 16 g/mol
Next, we can calculate the number of moles of methane contained in 32g of this compound:
16 g CH4 ----- 1 mol CH4
32 g CH4 ----- x
Solving for x, we have that there are 2 moles of methane and thus this is the amount that will be burned in this reaction.
Finally, knowing how many moles of methane will burn, we can calculate the amount of energy released from the molar enthalpy given:
1 mol CH4 ---------- 890 kJ of energy
2 mol CH4 ---------- y
Then, solving for y, we have that 1780 kJ of heat will be released when 32 g of methane are burned.