First, we have to analyze the meaning of the percentage m/m:
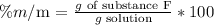
Then, we can calculate the required grams of the solution to carry out the reaction with substance F. We have to clear the equation in terms of the g solution as follows:
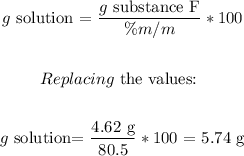
Now, let's convert the mass of the solution into volume, using the solution's density (1.23g/ml):
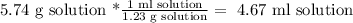
Then, the answer is that we need 4.67 ml of solution.