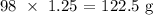Answer;
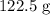
Explanation;
Here, we want to get the mass of the given number of molecules
Mathematically, 1 mole of a substance contains:
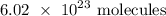
To get the number of moles in the given number of molecules, we divide the given number of molecules by the above
Mathematically, we have that as:
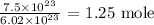
To get the mass, we have to multiply the number of moles above by the molar mass of the compound
To get the molar mass of the compound, we need to add the atomic masses of the constituent elements in the compound noting their counts
The atomic mass of hydrogen is 1 amu, the atomic mass of oxygen is 16 amu and the atomic mass of sulfur is 32 amu
Thus, we have the molar mass of the compound as:
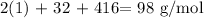
The molar mass of H2SO4 is 98 g/mol
Thus, the mass of 1.25 mole would be: