Answer:
The temperature change is 96.97°C.
Step-by-step explanation:
The given information from the exercise is:
- Mass (m): 150g
- Specific heat of mercury (c): 0.0330cal/g.°C
- Heat (q): 480cal
To calculate the temperature change (ΔT) it is necessary to use the Heat formula, and replace the values of mass, specific heat and heat:
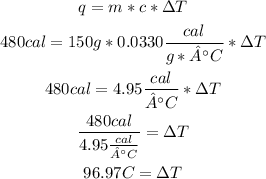
So, the temperature change is 96.97°C.