The mass of ammonia prepared from 24.5 kg magnesium nitride, according to the reaction Mg₃N₂(s) + 6H₂O(l) → 3Mg(OH)₂(s) + 2NH₃(g), knowing that the process is 71% efficient is 5.87 kg.
The balanced reaction of production of ammonia is:
Mg₃N₂(s) + 6H₂O(l) → 3Mg(OH)₂(s) + 2NH₃(g) (1)
First, let's find the number of moles of magnesium nitride
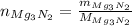 (2)
(2)
Where:
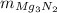 : is the mass of Mg₃N₂ = 24.5 kg
: is the mass of Mg₃N₂ = 24.5 kg
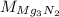 : is the molar mass of Mg₃N₂ = 100.9494 g/mol
: is the molar mass of Mg₃N₂ = 100.9494 g/mol
The number of moles is (eq 2):
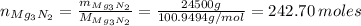
We can calculate the mass of ammonia prepared, knowing that 1 mol of Mg₃N₂ reacts with 6 moles of H₂O to produce 3 moles of Mg(OH)₂ and 2 moles of NH₃ (reaction 1).
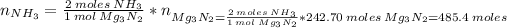
Then, the mass of NH₃ is:
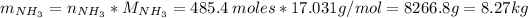
Since the process is 71% efficient, the mass that can be prepared is:
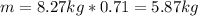
Therefore, the mass of ammonia that can be prepared is 5.87 kg.
I hope it helps you!