According to the Gay-Lussac's Law, if an ideal gas is at constant volume, then the quotient of its pressure and its temperature is also constant:
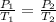
If the final temperature is 1.2 times the initial temperature, then, substitute T_2=1.2*T_1 and solve for P_2:
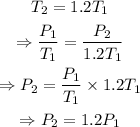
Therefore, the final pressure of the gas is 1.2 times the initial pressure.