Answer:
![\begin{gathered} a)\text{ Rate = k }*\text{ \lbrack NO\rbrack}^2\text{ }*\text{ \lbrack Cl}_2] \\ b)\text{ Order = 3} \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/e9r8c3fnlw3me4m7b3pgczuzc2qjyz6kdf.png)
Step-by-step explanation:
Here, we want to deduce the rate law for the reaction given
According to the data provided:
![Rate\text{ = k\lbrack NO\rbrack}^a[Cl_2]\placeholder{⬚}^b](https://img.qammunity.org/2023/formulas/chemistry/college/8mazpi06c4f00twtnkgr69haga9mz7ml00.png)
where the values in the square parentheses represent the concentrations and k represents the rate constant
Let us work with equations 1 and 3:
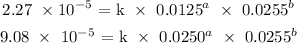
Divide equation 3 by 1:
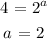
To get b, we can use equations 1 and 2:
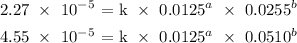
Divide equation 2 by 1, we have it that:
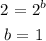
The rate law for the reaction is thus:
![Rate\text{ = k }*\text{ \lbrack NO\rbrack}^2\text{ }*\text{ \lbrack Cl}_2]](https://img.qammunity.org/2023/formulas/chemistry/college/w0c8dol7x1mgclu70spi7afeqimixgo5kr.png)
b) The overall order is the sum of the powers
That would be 1 + 2 = 3