Answer
The grams of H2 required = 3.0 grams
Step-by-step explanation
Given:
Mass of Fe2O3 = 80 g
Equation: 3H2 + 1Fe2O3 ----> 2Fe + 3H2O `
What to find:
The grams of H2 required to completely convert 80g of Fe2O3.
Step-by-step solution:
From the equation of reaction;
3 moles of H2 completely react with 1 mole of Fe2O3
Note: Molar mass of H2 is 2.016 grams per mole and Molar mass of Fe2O3 is 159.69 g/mol
This implies; (3 x 2.016 g) = 6.048 grams H2 completely react with 159.69 grams Fe2O3.
Therefore, x grams H2 will completely convert 80 grams Fe2O3.
Cross multiply and divide both sides by 159.69 grams Fe2O3.
x grams H2 is now equal to
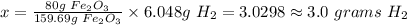
Hence, the grams of H2 required to completely convert 80g of Fe2O3 is 3.0 grams