To solve this question we have to convert the given mass of litium hydroxide to moles using the molecular mass of this compound:
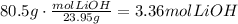
Now, use the stoichiometric ratio given by the chemical equation:
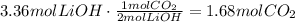
Finally, convert the moles of CO₂ to g using the molecular mass of CO₂:
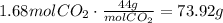
The mass of carbon dioxide required to react with 80.5g of LiOH is 73.92g.