Answer: the temperature of the gas under the conditions given is -266°C
Step-by-step explanation:
The question requires us to determine the temperature of a gas, in Celsius degrees, knowing that there are 2.49 moles of the gas contained in a 1.00 L vessel at a pressure of 143 kPa.
To solve this problem, we can apply a rearranged equation of ideal gases, as shown below:
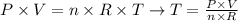
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles and R is the constant of gases.
Since the question did not provide a value for R, we'll apply 8.314 kPa.L/K.mol. Note that the temperature will be obtained in Kelvin, and we'll need to convert it to Celsius degrees.
Applying the values provided by the question, we'll have:
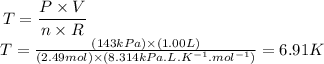
Therefore, the temperature of the gas is 6.91K.
We can convert this temperature to °C as it follows:
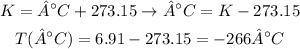
Therefore, the temperature of the gas under the conditions given is -266°C.