ANSWER:
c. directly proportional
Explanation:
We have that the equation of the ideal gases is the following:

Since pressure and temperature are constant, we would be left with:
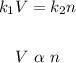
Which means that the relationship is directly proportional. So the correct answer is c. directly proportional