Step 1 - "Reading" the chemical equation
The given chemical equation is:
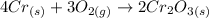
Let's read this equation. Remember: the numbers at the left of the substances represent the number of moles involved in this reaction.
Thus:
4 moles of Cr react with 3 moles of O2 to produce 2 moles of Cr2O3
As the exercise is specifically asking about the relation between O2 and Cr2O3, we can further simplify this statement to:
3 moles of O2 produce 2 moles of Cr2O3
This is a fixed relation we'll be using to solve the problem.
Step 2 - How many moles of C2O3 will be produced?
We know that 3 moles of O2 produce 2 moles of Cr2O3. Since the exercise gave us a mass of O2, 1.34x10^3 g, it is convenient to convert moles of O2 to mass.
In order to so, we need to multiply the number of moles by the molar mass (32 g/mol for O2):

Therefore, 96g of O2 are required to produce 2 moles of Cr2O3. We can use this relation to predict how much C2O3 will be produced:
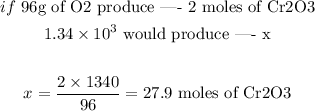
Therefore, 27.9 moles of Cr2O3 would be produced.
Step 3 - Converting number of moles of Cr2O3 to number of molecules
One mole corresponds to 6.10^23 unities. Therefore, to convert the number of moles of Cr2O3 to number of molecules, we can set the following proportion:
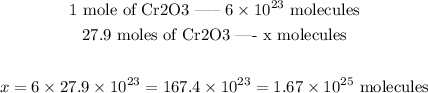
Answer: 1.67*10^25 molecules of Cr2O3 would be produced in this reaction.