Step 1: balance the equation.
The equation given is already balanced
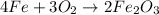
Step 2: Calculate the number of moles of Fe
n = m/M
n = 34.7 g/55,845 g/mol
n = 0.621 mol
Step 2: Use the stoichiometry to find moles of O2
The molar rato between Fe and O2 is 4:3
Therefore the number of moles O2 = 0.621 x (3/4) = 0.466 mol
The answer is 0.466 mol.