Answer
19.93 mol
Step-by-step explanation
Given:
The number of particles of CN = 1.2 x 10²⁵
What to find:
The number of moles of CN present in 1.2 x 10²⁵ particles of CN.
Step-by-step solution:
According to Avogadro, 1 mole of CN has 6.022 x 10²³ particles.
Therefore, the number of moles of CN present in 1.2 x 10²⁵ particles of CN will be:
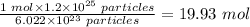
The number of moles of CN present in 1.2 x 10²⁵ particles of CN = 19.93 mol.