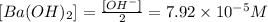pH is a symbolic representation of:
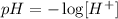
and for water we now that:
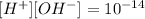
that means the text give us the information to calculate the concentration of protons and therefore the concentration of ions OH-:
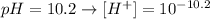
therefore the concentration of OH- can be calculated:
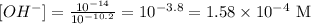
but they are not asking about the concetration of OH- they ask about concentration of Ba(OH)2 and for that we need to know the disolution stechiometry
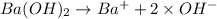
Whic mean the concentration of Ba(OH)2 is half the concentration of OH-: