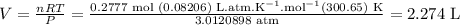Answer
2.27 L
Procedure
The volume occupied by one mole of any gas at standard temperature(0°C) and pressure(1 atm) is 22.4 liters.
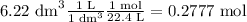
Then we will use the ideal gas formula to get the volume.
PV=nRT
Where R= 0.08206 L⋅atm⋅°K⁻¹⋅mol⁻¹
P= pressure
V=volume
n= moles
T= temperature in °K
Solving for V