Answer:
BaSO4.
Step-by-step explanation:
What is given?
If we assume that the total mass of the compound is 100 g:
Mass of barium (Ba) = 58.8 g.
Mass of sulfur (S) = 13.7 g.
Mass of oxygen (O) = 27.5 g.
Molar mass of Ba = 137 g/mol.
Molar mass of S = 32 g/mol.
Molar mass of O = 16 g/mol.
Step-by-step solution:
The first step is to calculate the number of moles of each element using their respective molar mass. Let's start with Ba:
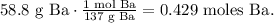
For S:
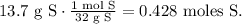
And for O:
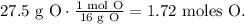
The next step is to divide each number of moles by the least number of moles obtained. In this case, the least number of moles obtained was 0.428 moles:
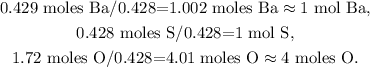
As we obtained 1 mol of Ba, 1 mol of S, and 4 moles of O, the empirical formula would be BaSO4.