Answer
Step-by-step explanation
Given:
Volume, V = 212 L
Mass of argon = 23300 g
Temperature, T = 25°C = (25 + 273.15 K) = 298.15 K
What to find:
The pressure in the tank.
Step-by-step solution:
The pressure in the tank can be calculated using the ideal gas equation which is:

n is the number of moles and can be also be:
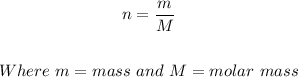
Put n = m/M into the ideal gas equation above:
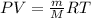
The molar mass of argon = 39.948 g/mol
Put the values of the parameterss into the formula to get pressure, P:3
![undefined]()