Step 1 - Which equation should we use?
Note the exercise is asking us the pH, but gave instead the concentration of OH- ions. That's not a problem, since pH and pOH are related through the following equation:
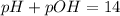
Therefore, if we discover the pOH, we can also find the pH of a solution.
Step 2 - Calculating the pOH of the solution
The definition of pOH is as follows:
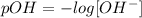
The concentration of OH- in the given solution is 5.5*10^-4 mol/L, so:
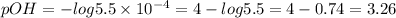
Step 3 - Finding the pH of the solution
Now we can find the pH:
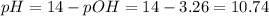
Answer: the pH is equal to 10.7