To calculate the calcium particles that are needed we must take into account Avogadro's number, this number tells us that in one mole of any substance there are 6.022x10^23 particles.
So if we have 3 moles of Calcium (Ca), we must multiply this value by 3 to obtain the number of particles of Ca, that is, we apply the following operation.
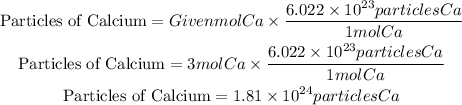
To have 3.00 moles of Calcium will be required 1.81x10^24particles of Calcium