We are given :
• Concentration of HCL = ,0.500 M
,
• Volume of lead(ii) nitrate = 2.5 L = 2.5 *1000 = ,2500 ml
,
• Density of leadii) nitrate =, 4.53g/L
,
• Volume of HCL =?
1. Calculate mass of lead(ii) nitrate :
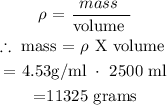
2. Calculate moles of lead (ii) nitrate :
• Molecular mass of lead(ii) nitrate = 331,2 g/mol
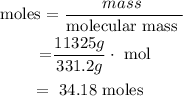
3. Calculate moles of HCL
Soichemistry chemical equation shows that :
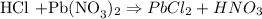
• meaning : 1 mole of HCL reacts with 1 mole of Pb(NO3)2
,
• Therefore 34.18 moles HCL will react with 34.18 moles Pb(No3)2
,
• number of moles of HCL = 34.18
4. Calculate volume of HCl needed
• Concentration HCl = 0.5 M and molesHCl = 34.18
![undefined]()