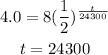Given data
*The half-life time of polonium-239 is T = 24,300 years
*The nuclear bomb released the isotope is N = 8 kg
*The reduced amount is N(t) = 4.00 kg
From the radioactive decay equation, it is given as
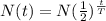
Substitute the values in the above expression as