Answer
The molarity of the KOH = 1.542 M
Step-by-step explanation
The given parameters are:
Volume of KOH, Vb = 50.0 mL
Volume of H2SO4, Va = 25.7 mL
Molarity of H2SO4, Ca = 1.50 M
Equation: 2KOH(aq) + H2SO4(aq) → K2SO4(aq) + 2H2O(l)
From the equation, the mole ratio of KOH to H2SO4 = 2:1, that is na = 1 and nb = 2
What to find:
The molarity of the KOH, Cb.
Step-by-step solution:
The molarity of the KOH, Cb can be calculated using the formula below:
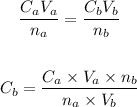
Substituting the values of the parameters into the formula, we have

Thus, the molarity of the KOH is 1.542 M