Let's first copy the reaction:
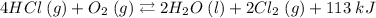
By Le Chatelier's Principle, When we change something in the system, the equilibrium shifts so it minimizes the effect of the change.
Let's see what happens with each option:
1) an increase in pressure mostly affect the gases. On the left side, we have a total of 5 of gases involved (4 HCl and 1 O₂), while on the right side, we have only 2 gases (2 Cl₂). An increase in pressure would be answer with a shift that minimizes this change. The more gas we have, the higher is the pressure, so the equilibrium would change so that we have less gas, thus, less pressure. The side with less gases is the right side, so the products, thus the equilibrium would shift for the formation of more produtcs.
2) An increase in temperature affect the energy involved on the equilibrium. If we have higher temperature, we have more energy available, so the equilibrium is shifted so we consume this energy we have increased. Since th right side has energy being released, the equilibrium would shift to consume this energy, so it would shift for the formarmation of more reactants
3) HCl is one of the reactants of the equilibrium, since we decreased it, the equilibrium would shift so we produce more of it, so it will shifts for the production of more reactants.
4) Cl₂ is a product, so an increase in it will shift so that more Cl₂ is consumed, so it will shifts for the production of more reactants.
So, the only option that would drive the equilibrium for the formation of more products is 1.