Given:
A(2, -1), B(5, -1), C(5, 3)
Let's determine the if the triangle ABC is a right triangle.
To determine if it is a right triangle, let's find the length of each side by using distance formula, then apply Pythagorean Theorem.
Apply the distance formula:
![d=\sqrt[]{(x2-x1)^2+(y2-y1)^2}](https://img.qammunity.org/2023/formulas/mathematics/college/1h551ypq5weta3sw0dynfch7nxiwrgmnba.png)
Thus, we have:
Length of AB:
A(2, -1), B(5, -1)
![\begin{gathered} AB=\sqrt[]{(5-2)^2+(-1-(-1))^2} \\ \\ AB=\sqrt[]{(3)^2+(0)^2}=\sqrt[]{3^2}=3 \end{gathered}](https://img.qammunity.org/2023/formulas/mathematics/college/7r0pvrkh5c0lshsizpxonczji523owh3l1.png)
Length of BC:
B(5, -1), C(5, 3)
![\begin{gathered} BC=\sqrt[]{(5-5)^2+(3-(-1))^2} \\ \\ BC=\sqrt[]{(0)^2+(3+1)^2}=\sqrt[]{0+4^2}=4 \end{gathered}](https://img.qammunity.org/2023/formulas/mathematics/college/fj0qp9cmbipxxj11apyxy41atvjx1y6db1.png)
Length of AC:
A(2, -1), C(5, 3)
![\begin{gathered} AC=\sqrt[]{(5-2)^2+(3-(-1))^2} \\ \\ AC=\sqrt[]{(3)^2+(3+1)^2} \\ \\ AC=\sqrt[]{9+16}=√(25)=5 \end{gathered}](https://img.qammunity.org/2023/formulas/mathematics/college/b6d3pttplwl8q13y3j7mdwf9y5yf0m6v3x.png)
Thus, we have the following side lengths of triangle ABC:
AB = 3
BC = 4
AC = 5
APply Pythagorean Theorem:
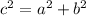
Where:
a = 3
b = 4
c = 5
Let's verify if the equation is true:
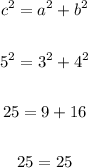
SInce the left hand side equals the right hand side, the equation is true.
Therefore, the triangle ABC is a right triangle.
ANSWER:
Yes, the triangle ABC is a right triangle.