Answer:
Step-by-step explanation:
The question requires us to identify the excess reactant and the amount of excess if 72g of CS2 are reacted with 52g of O2, considering the following chemical equation:
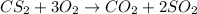
To solve this problem, we'll need to consider the stoichiometry of the reaction and calculate how many moles of CS2 would be necessary to react with the given amount of O2 (or, alternatively, how many moles of O2 would be necessary to react with the given amount of CS2).
First, let's convert the masses given in number of moles of the substances.
Knowing that the molar mass of CS2 is 76.14 g/mol and the molar mass of O2 is 31.98 g/mol, we can calculate the number of moles used for each of these substances:
- For CS2:
76.14g CS2 -------------------- 1 mol
72g CS2 ----------------------- x
Solving for x, we have that 72g of CS2 corresponds to 0.94 moles of CS2.
- For O2:
31.98g O2 --------------------- 1 mol
52g O2 ------------------------ y
Solving for y, we have that 52g of O2 corresponds to 1.6 moles of O2.
Now, we can use the stoichiometry of the reaction to determine the excess reactant.
From the balanced chemical equation, we can see that 3 moles of O2 are necessary to react with 1 mol of CS2. Thus, we can calculate how many moles of O2 would be necessary to react with 0.94 moles of CS2:
1 mol CS2 --------------------------- 3 mol O2
0.94 mol CS2 -------------------- a
Solving for a, we have that 2.83 moles of O2 would be necessary to react with the given amount of CS2.
Alternatively, we can calculate how many moles of CS2 would be necessary to react with 1.6 moles of O2:
1 mol CS2 --------------------------- 3 mol O2
b --------------------------------------- 1.6 mol O2
Solving for b, we have that 0.59 moles of CS2 would be necessary to react with 1.6 moles of O2.
Therefore, considering the stoichiometry of the reaction, 2.83 moles of O2 would be required to react with the given amount of CS2 - since the amount of O2 given is smaller (1.6 moles), we can say that O2 is the limiting reactant.
Also, we calculated that 0.59 moles of CS2 would be necessary to react with the given amount of O2 - since the amount of CS2 given is greater (0.94 moles), we can say that CS2 is the excess reactant.
At last, we can calculate the amount of excess.
If 0.59 moles of CS2 would be required but 0.94 moles of it were used, we have:
excess of CS2 = (0.94 - 0.59) mol = 0.35 mol CS2
To calculate the mass correspondent, we use the molar mass of CS2 (