Since the sun is almost directly overhead, then we can say that we have two right triangles that are similar.
So, we can formulate the following equation:

The height is 15.21 ft.
Converting the answer to inches, we have:
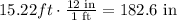
The answer is 183 inches (Rounding to the nearest inch)