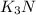Answer:
Step-by-step explanation:
Here, we want to get the empirical formula of the compound with the given data
We start by dividing the given percentages by the atomic masses of the given elements
The atomic mass of Potassium is 39 amu
The atomic mass of Nitrogen is 14 amu
Proceeding with this, we have it that:
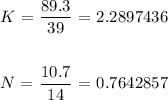
From here, we have to divide each of the values by the smaller value from the above division
We have that as:

Thus, we have the empirical formula of the compound as: