Step 1 - Finding the molar mass of glycine
The molar mass of glycine can be found by multiplying the molar mass of each element by the number of times it appears in the formula and then summing it all up.
For glycine, we find the molar mass of 75.07 g/mol.
Step 2 - Interpreting the meaning of molar mass
The molar mass gives us the total mass of one mole of that substance. Let's take water as an example: its molar mass is 18 g/mol, which means one mole of water would weight 18 g, two moles 36 g and so on.
For glycine, therefore, one mole weights 75.05 g.
Step 3 - Finding the number of moles
To find the number of moles, we can use a very simple formula relating mass (m), molar mass (M) and number of moles (n):
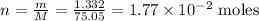
Therefore, there are 1.77*10^(-2) moles in 1.332 g of glycine.