To solve this problem we have to use the rule of dilutions:
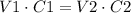
Where V1 is the initial volume, C1 is the initial concentration, V2 is the final volume and C2 is the final concentration.
Replace V1 for 0.560L, C1 for 43.6% and C2 for 20.4% and solve the equation for V2:
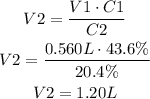
It means that the final volume is 1.20L.