Answer:
66.58g of oxygen gas will produce 141.43g of Al2O3.
Step-by-step explanation:
1st) It is necessary to write the balanced chemical equation:
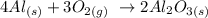
From the balanced chemical equation we know that 4 moles of Al react with 3 moles of Oxygen to produce 2 moles of Al2O3.
With the molar mass of Al (26.98g/mol), O2 (32g/mol) and Al2O3 (101.96g/mol), we can convert the number of mole to grams:
So, 107.92g of Al react with 96g of O2 to produce 203.92g of Al2O3.
2nd) With the given value of Al2O3, and the balanced chemical equation we can calculate thr grams of O2 that will be produced:
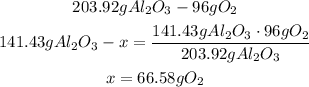
So, 66.58g of oxygen gas will produce 141.43 grams of Al2O3.