First, we need the reaction:
2N2 (g) + O2 (g) → 2N2O (g)
Molecular weights:
N2 = 28.01 g/mol
N2O = 44.01 g/mol
For this exercise we use Stoichiometry.
Always balance your reaction.
---------------------------------------------------------------------------------
We know that:
2 x 28.01 g N2 -------------------- 2 x 44.01 g N2O
X -------------------- 5.80 g N2O
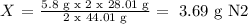
Answer: 3.69 grams of N2 are needed.