Answer:
The new pressure is 3.94atm.
Step-by-step explanation:
1) The law of gases when the temperature is constant is called Boyle's law.
2)
• Identify the given
The given information is:
P1: 2.5 atm (initial pressure)
V1: 850mL (initial volume)
V2: 540 mL (final volume)
P2: this is what we have to calculate (final pressure).
• Equation, of Boyle's law
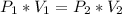
• Resolution
We have to replace the given values to calculate the final pressure (P2):
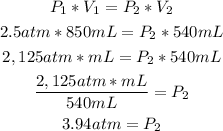
So, the new pressure is 3.94atm.