Answer: volume of gas at 175°C = 18.10mL.
Explanations
Given :
• Temperature (T1 = 175°C
,
• Volume (V1 ) = 132 mL
,
• Temperature (T2) = 24 °C
,
• Volume 2 = ....mL?
We will consider Charles law "volume of an ideal gas at constant pressure is directly proportional to the absolute temperature"
• Represented as :
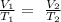
Replacing the given parameters into chales law equation , we get that V2 =
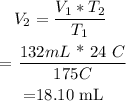
This means that the volume of gas at 175 = 18.10mL.