Given:
The mass of water is: m = 62 g = 0.062 kg
The change in temperature is: ΔT = 356 K - 273 K = 83 K
The specific heat capacity of water is: c = 4185.5 J/kgK
To find:
The amount of heat required to raise the temperature of 62g of water of from 273 K to 356 K.
Step-by-step explanation:
The amount of heat Q required to raise the temperature of mass m of a substance is given as:
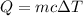
Here, c is the specific heat capacity and ΔT is the change in the temperature of the substance.
Substituting the values in the above equation, we get:
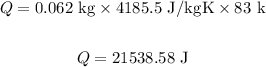
Final answer:
The amount of heat required is 21538.58 J.