Answer
Step-by-step explanation
Given that:
What to find:
The coefficient of H2O in the balanced oxidation half-reaction.
Solution:
Step 1: Identify the species undergoing oxidation and reduction.
Mn changes from +7 in MnO₄⁻ to +6 in MnO₄²⁻. It is undergoing a reduction
S changes from +4 in HSO₃⁻ to +6 in SO₄²⁻. It is undergoing oxidation.
Step 2: Write the half-reaction for oxidation and reduction.
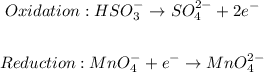
Step 3: Balance the half-reactions by equating the number of electrons on both sides.
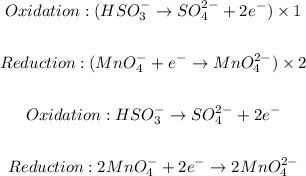
Step 4: The number of electrons will be canceled so the overall reaction will be:
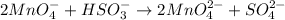
Step 5: Balance the number of Oxygen atoms by adding H₂O on the left side.
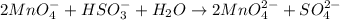
Step 6: Balance the number of H by adding H⁺ (acid) on the right side.