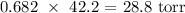Answer:
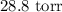
Step-by-step explanation:
Here, we want to get the vapor pressure of the solution
Firstly, we need to get the mole fraction of water
That would be the number of moles of water divided by the sum of the number of moles of all
We have that as:
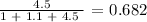
According to Raoult's law:
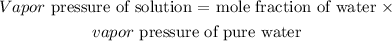
Thus, we have the vapor pressure of the solution as: