We have here an exponential decay. In this case, we have to use the formula:
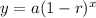
Where:
a = initial value (in this case, 360 grams)
r = decay rate (in this case, we have 50/100= 0.5.
x = number of time intervals that have passed (in this case, 4 hours).
Then, we have that:
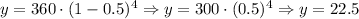
Hence, after four hours there will be 22.5 grams of the radioactive material.