We have to use the expected value formula, which is about the sum of multiplying each event by its probability.
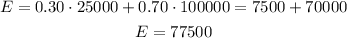
Given that David didn't purchase the insurance, we don't have to include that information in the formula.
Therefore, the expected net profit is $77,500.