To go from moles to molecules we will apply Avogadro's law. Avogadro's law tells us that one mole of any substance contains 6.022x10^23 molecules. So applying this relationship, the calcium chlorate molecules will be:
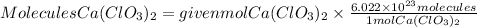
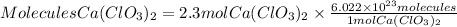
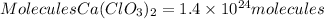
In 2.3 moles of calcium chlorate, there are 1.4x10^24 molecules