Answer
102.816 liters
Step-by-step explanation
Given:
Number of moles = 4.59 moles
At STP, one mole of any gas occupies a volume of 22.4 L
This implies;
1 mole NO₃ = 22.4 L
4.59 moles NO₃ will occupy x L
So, x will be:
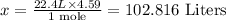
102.816 liters of NO3 are in 4.59 moles of NO3