Density is a property of matter that relates mass to volume, the equation that describes it is as follows:
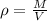
Where,
M is the mass of the substance
V is the volume of the substance
Now, to calculate the density we are missing the mass of the added milk, but we can find it if we subtract the mass of the glass from the total mass (mass of the drinking glass plus the milk). The mass of milk will be:
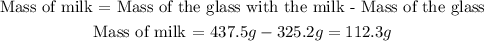
Now that we have the mass of milk we can calculate the density:
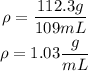
The density of the milk is 1.03g/mL