This problem is about the area of a square.
Notice that the mat has square form, this means its area is defined by
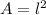
Where l is the length of each side.
Now, to find the length of the side, we just need to use the formula above and replace the area with its number 81
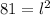
We solve for l
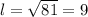
Therefore, the length of the side is 9 feet.