Answer
0.0972
Step-by-step explanation
Given:
Mass of calcium oxide = 5.45 grams
What to find:
The number of moles for the mass.
Step-by-step solution:
The number of moles in 5.45 grams CaO can be calculated using the mole formula.
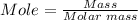
From the periodic table, the molar mass of CaO = (40.078 + 15.999) = 56.077 g/mol
Therefore,
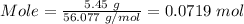
The number of moles for the mass of 5.45 grams CaO = moles