Given data:
* The initial internal energy of the car is E_i = 2769 J.
* The amount of work done on the car to accelerate is dW = 527 J.
* The final internal energy of the car is E_f = 3228 J.
Solution:
From the first law of thermodynamics, the heat energy loss by the car is,
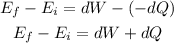
Where the negative sign indicates the loss of heat energy from the car,
Substituting the known values,

Thus, the amount of energy loss by the car in form of heat is -68 J.